XADAGO clinical
efficacy & safety

XADAGO clinical efficacy & safety
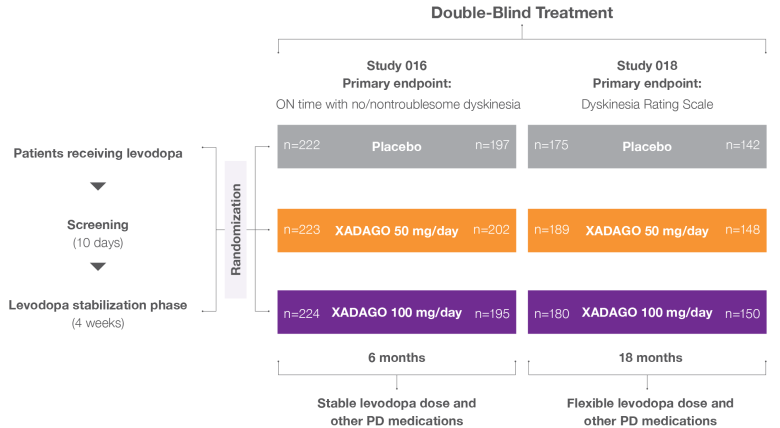
XADAGO 6-month study designs1-3
Two pivotal, 6-month studies: Study 1 (016) and Study 2 (SETTLE)
In 2 randomized, 24-week, multicenter, double-blind, placebo-controlled trials, the efficacy and safety of XADAGO as add-on therapy to a stable dose of levodopa in patients with Parkinson’s disease (PD) experiencing motor fluctuations were evaluated. After a levodopa stabilization phase, patients were randomized to receive XADAGO 50 mg/day or 100 mg/day or placebo.
Study 1 (016) and Study 2 (SETTLE) efficacy outcomes
Primary endpoints
The primary endpoints were mean daily ON time (ON time without dyskinesia plus ON time with nontroublesome dyskinesia) at 6 months
Secondary endpoints
Secondary outcomes of Study 1 and Study 2 included:
- Decrease in total daily OFF time, as measured by Hauser diary
- Unified Parkinson’s Disease Rating Scale (UPDRS) Part III during ON phase—mean change from baseline to end of study
- Parkinson’s Disease Questionnaire 39 (PDQ-39): mean change from baseline to end of study
In Study 2, if there were no tolerability issues by day 14, the starting dosage was increased to 100 mg/day3
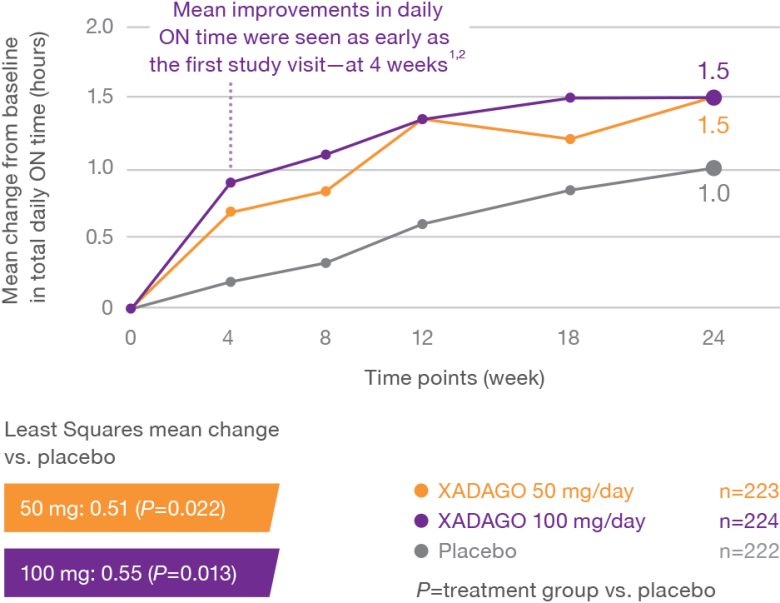
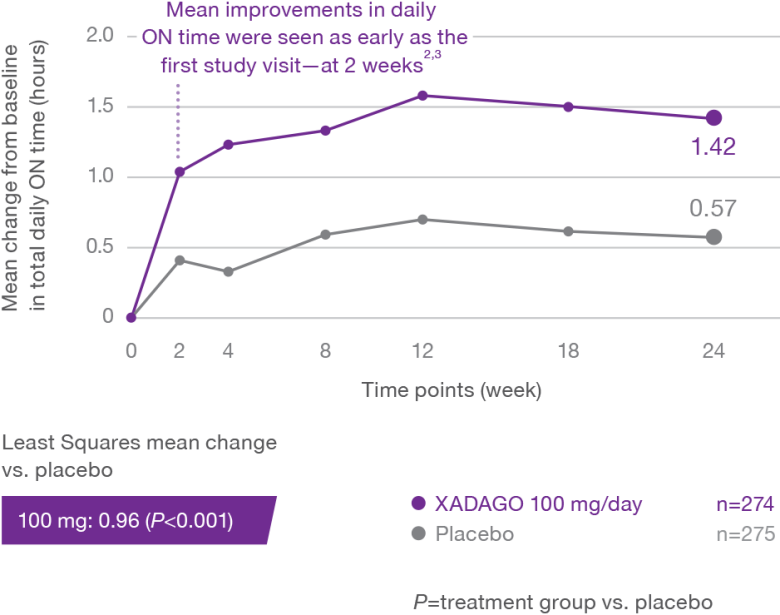
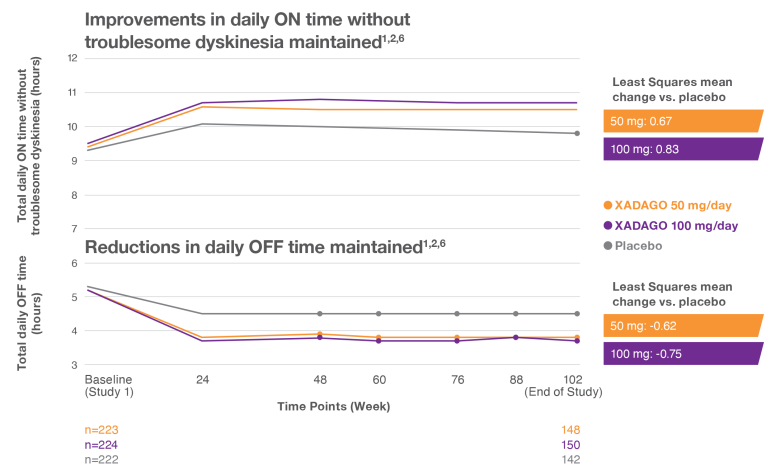
More daily ON time—without troublesome dyskinesia1-3
At 6 months, XADAGO significantly increased daily ON time without troublesome dyskinesia when used as an adjunct to levodopa1-3
Study 1 (016)1,2
Study 2 (SETTLE)2,3
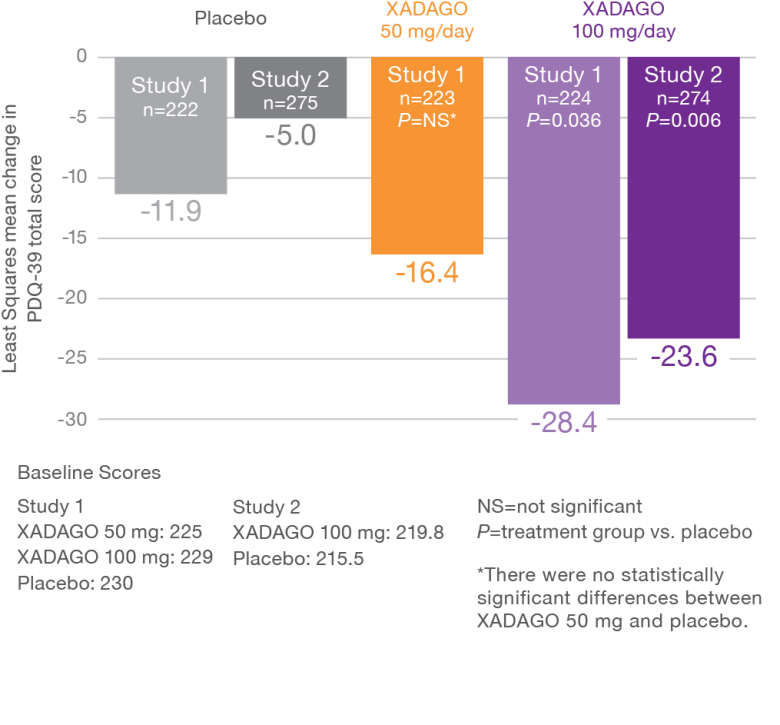
XADAGO patients reported significant improvements in their health status at 6 months1-3
XADAGO 100-mg dose group showed significant improvements based on PDQ-39 total scores1-3
PDQ-39 is a PD-specific health status questionnaire comprising 39 items. Items are grouped into 8 subscales (mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, bodily discomfort) that are scored by expressing summed item scores as a percentage score ranging between 0 and 100.4
Study 1 (016) and Study 2 (SETTLE)1-3
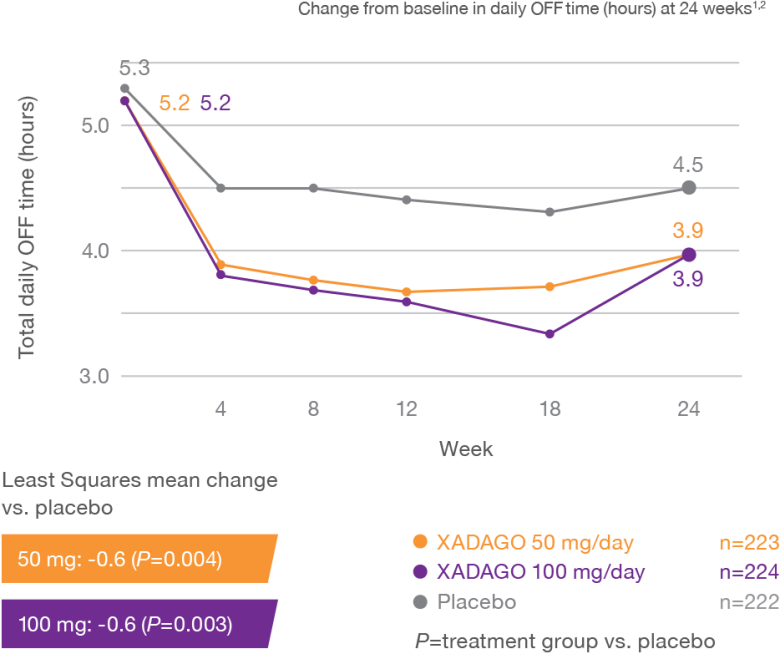
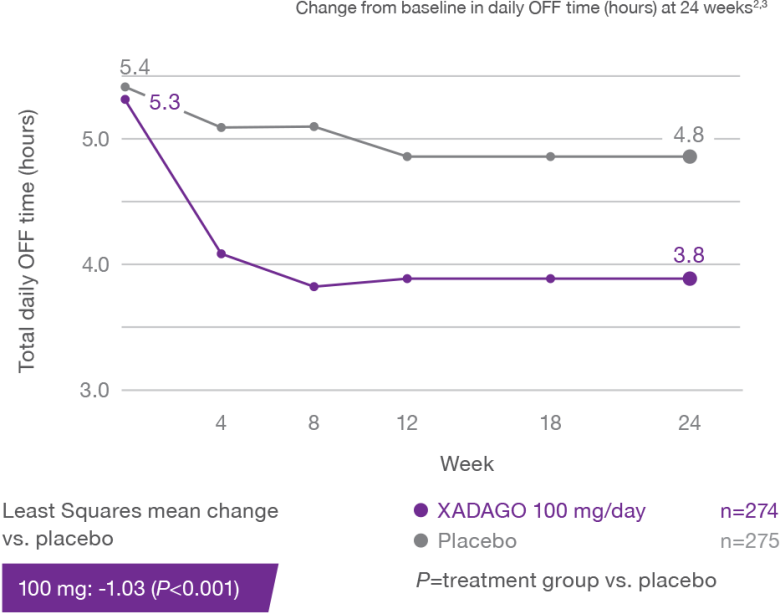
XADAGO significantly reduced daily OFF time at 6 months1-3
Study 1 (016)
Study 2 (SETTLE)
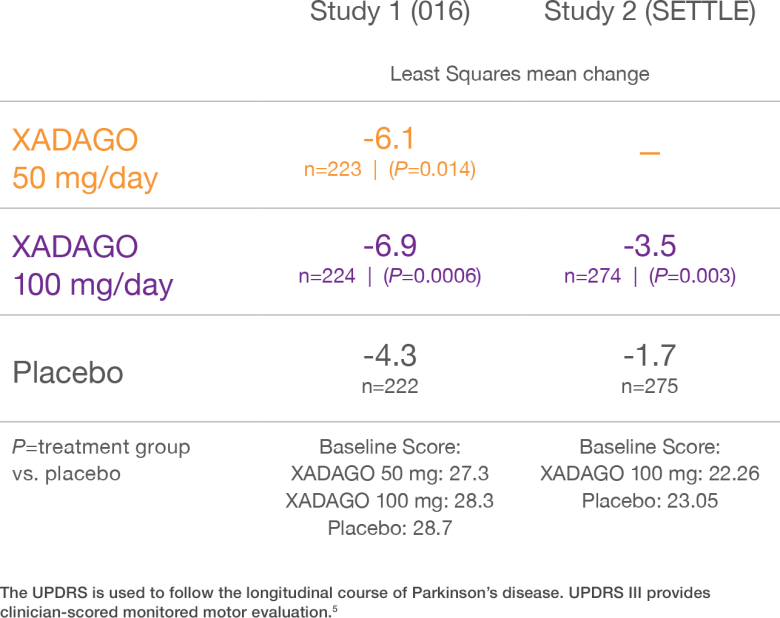
XADAGO improved motor function at 6 months based on UPDRS III scores1-3
Change from baseline in UPDRS III score during ON time1-3
18-Month extension to Study 1 (016)1,2,6
Study 018 was a double-blind, placebo-controlled, 18-month extension to Study 1 (016), aimed at assessing the long-term efficacy and safety of XADAGO as add-on therapy to levodopa in patients with PD and motor fluctuations
The primary efficacy endpoint in Study 018 was the Dyskinesia Rating Scale (DRS) score and was not statistically significant
Secondary outcomes of Study 018 included:
ON time and OFF time improvement maintained over 2 years1,2,6
The primary efficacy endpoint in Study 018 was the DRS score and was not statistically significant
Safety profile
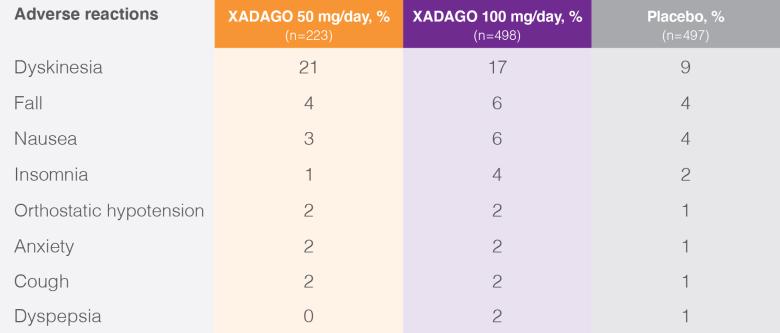
Patients with adverse reactions with an incidence ≥2% in the XADAGO 100 mg/day group and greater than placebo in studies 1 and 27
Discontinuation due to adverse reactions7
Overall discontinuation rates due to adverse reactions from Study 1 (016) and Study 2 (SETTLE) were 5% for XADAGO 50 mg/day, 6% for XADAGO 100 mg/day, and 4% for placebo7
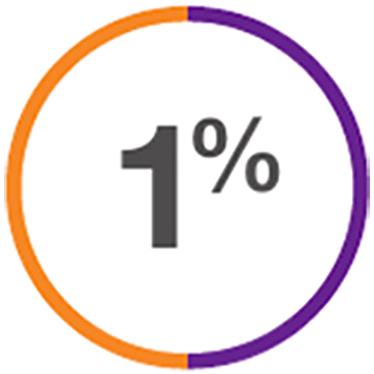
Discontinuation due to dyskinesia*,7:
for both XADAGO 50 mg/day and 100 mg/day dosages
*Discontinuation due to dyskinesia for placebo was 0%.
Medical inquiries and reporting adverse events
- For all medical inquiries, contact Supernus Pharmaceuticals Medical Affairs: 1-888-492-3246 or medinfo.supernus@apcerls.com. To report SUSPECTED ADVERSE REACTIONS or product complaints, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
- You may also report SUSPECTED ADVERSE REACTIONS or product complaints to Supernus Pharmaceuticals at 1-888-4XADAGO (1-888-492-3246)
- Additional information can be requested using the Supernus Pharmaceuticals Information Request Form